Topics
The program will cover the following topics:
- Environmental fate and ecological characteristics of drugs
- Artificial intelligence in pharmaceutical analysis
- Novel methodologies for drug analysis
- Biophysical and biological quality control techniques for medicines
- Sampling and sample preparation (including non-invasive analytical techniques)
- Pharmaceutical process data analysis (multivariate approaches, fingerprinting, chemometrics, QbD)
- Pharmaceutical regulatory quality (including novel guidelines, counterfeiting, vaccines, etc)
- Natural product pharmaceuticals
- Micro and nanotools in pharmaceutical analysis (SERS, nanofluidics, nanomagnets, single cell analysis, etc.)
- Omics for personalized medicine (microbiome, genomics, metabolomics, proteomics, peptidomics, lipidomics, venomics, etc.)
- Drug discovery and development (including MS and imaging in ADME studies)
- Biopharmaceuticals and Advanced Therapy Medicinal Products (bioanalysis, metabolism)
- Biomedical and diagnostic applications (including biomarkers, biosensors, and in vitro diagnostic)
- Affinity and bioactivity (ligand binding analytics)
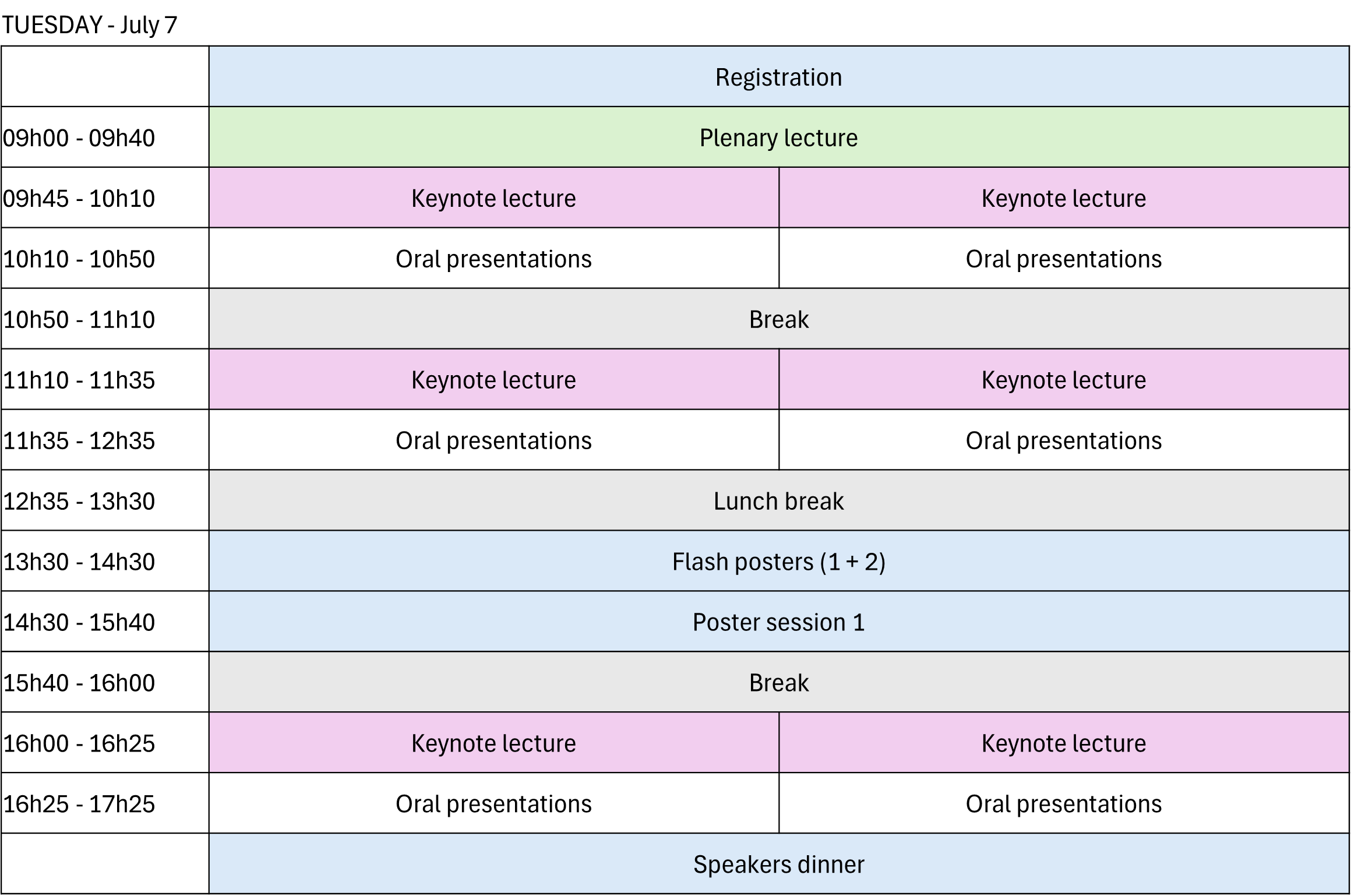
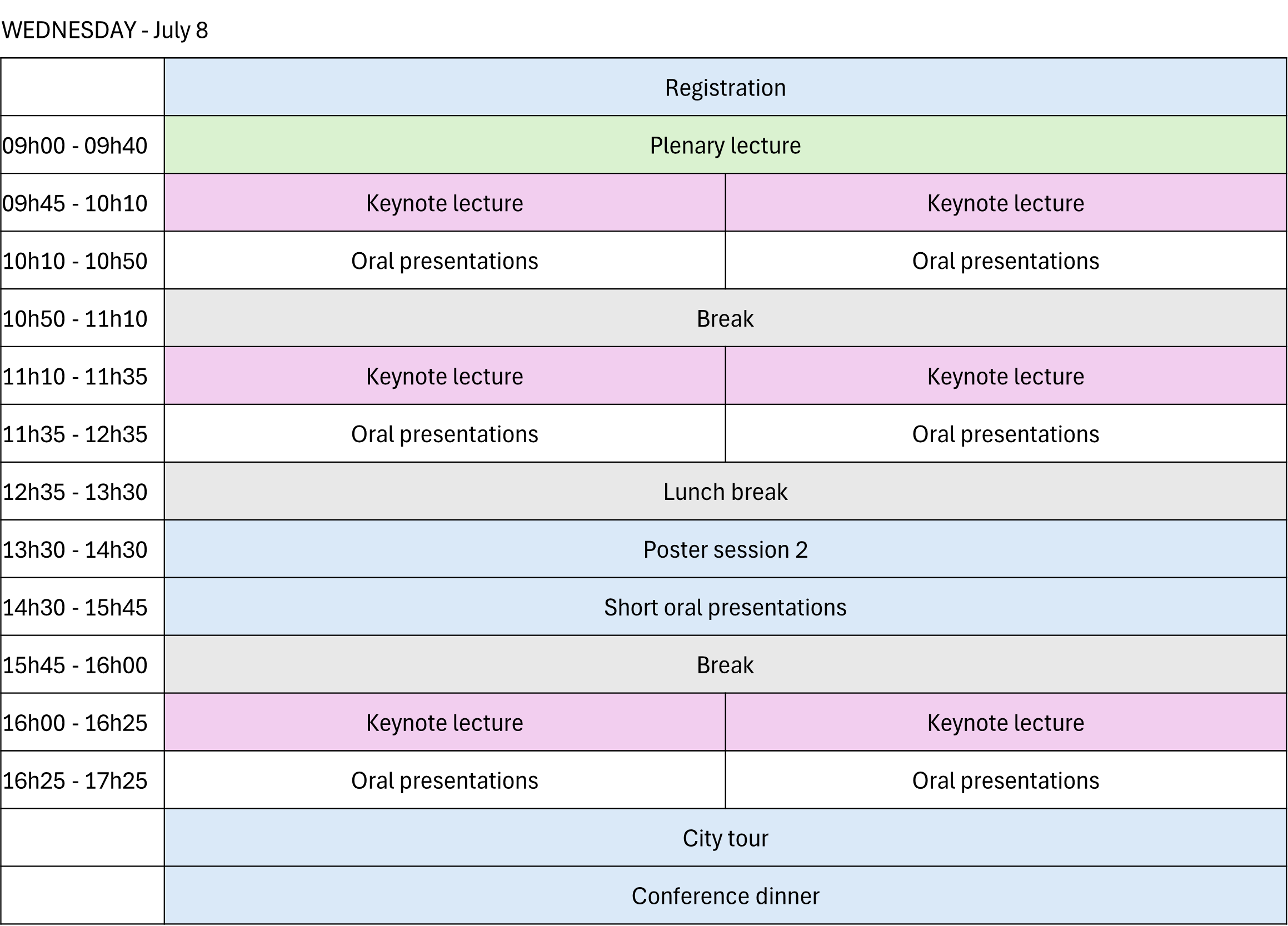
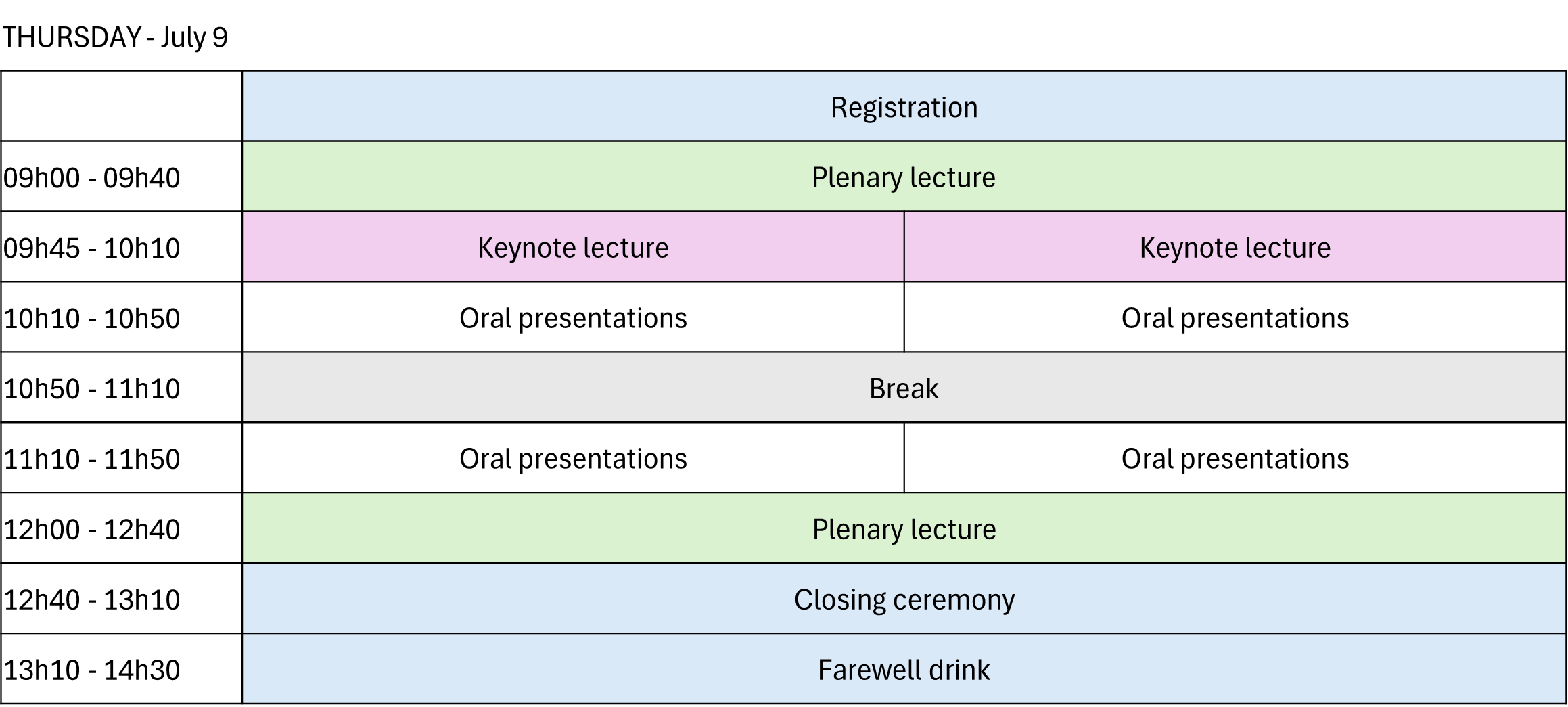
Parallel sessions will be organized, with one generally oriented toward bioanalysis and the other toward pharmaceutical analysis.
Short courses
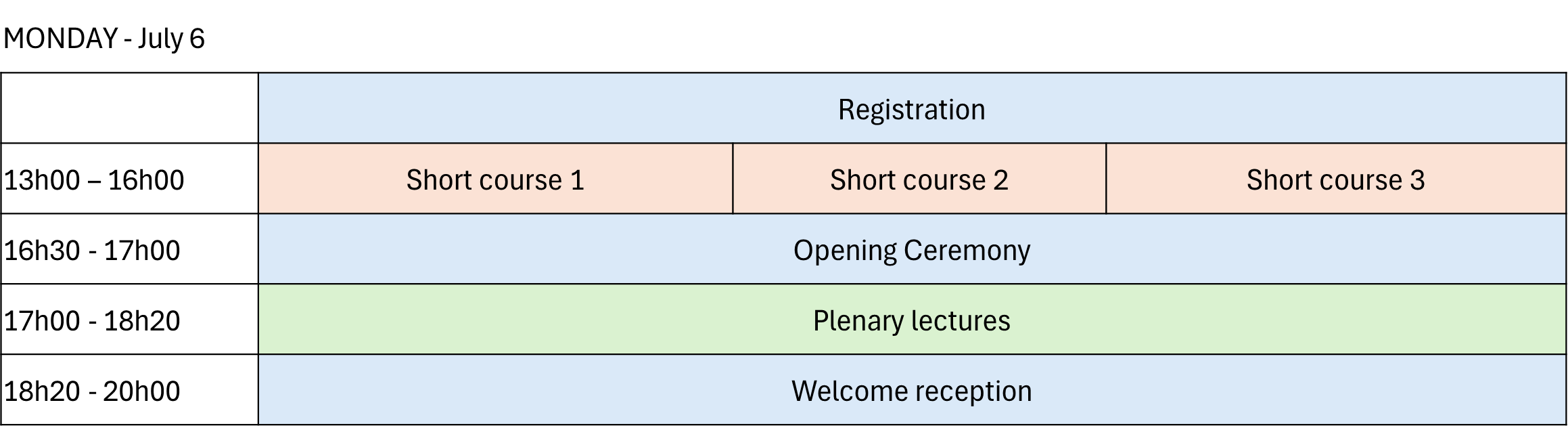
Half-day courses will be held on Monday, 6 July 2026, prior to the official opening of the conference.
The following half-day courses are offered:
- Analytical Quality by Design (QbD) for ATMPs
- The role of chromatography and mass spectrometry in protein biopharmaceutical analysis
- Challenges in liquid chromatography
Register for these courses: click here.
Preliminary programme